Bovine IL-17A Do-It-Yourself ELISA, ≤10 Plates
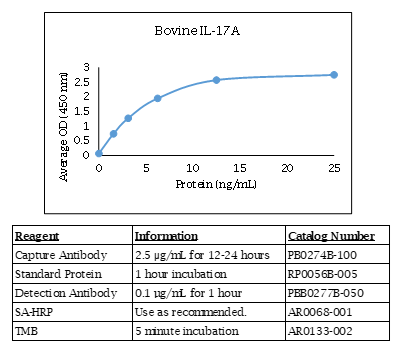
Bovine IL-17A ELISA Data
Bovine IL-17A ELISA Kit Components
| Component | Usage | Quantity | Catalog # |
| Anti-Bovine IL-17A Polyclonal Antibody | Capture Antibody | 100 µg | PB0274B-100 |
| Biotinylated Anti-Bovine IL-17A Polyclonal Antibody | Detection Antibody | 50 µg | PBB0277B-050 |
| Bovine IL-17A Recombinant Protein | Standard | 5 µg | RP0056B-005 |
Specifications
The Bovine IL-17A Do-It-Yourself ELISA contains capture antibody, protein standard, and detection antibody for development of a Bovine IL-17A ELISA. The antibodies have been determined to function in an ELISA with the standard provided. Optimal buffers, concentrations, incubation times, incubation temperatures, and methods for the ELISA have not been determined. A working knowledge of ELISA is strongly recommended. The quantities of components provided are not matched. Components may also be purchased separately.
The Bovine IL-17A Do-It-Yourself ELISA can also be used to measure Zebu IL-17A.
For additional tips and techniques to ensure a successful ELISA, check out our ELISA Technical Guide.
Background
IL-17A is a member of the IL-17 family, which is comprised of 6 members [IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F]. IL-17 family members are involved in numerous immune regulatory functions, including inducing and mediating proinflammatory responses and allergic responses. IL-17 induces the production of many other cytokines (IL-6, G-CSF, GM-CSF, IL-1β, TGF-β, and TNF-α), chemokines, including IL-8 (CXCL8), GRO-α (CXCL1) and MCP-1 (CCL2) and prostaglandins from many cell types (fibroblasts, endothelial cells, epithelial cells, keratinocytes and macrophages).
Alternate Names - IL17A, CTLA8, IL-17, IL-17A, IL17, CTLA-8, interleukin 17A
Defining immune correlates during latent and active chlamydial infection in sheep
Wattegedera SR, Livingstone M, Maley S, Rocchi M, Lee S, Pang Y, Wheelhouse NM, Aitchison K, Palarea-Albaladejo J, Buxton D, Longbottom D, Entrican G.
Vet Res. 2020 Jun 1;51(1):75. doi: 10.1186/s13567-020-00798-6.
Applications: Measurement of bovine IL-17A in cell culture supernatants by ELISA
Characterization of local and circulating bovine γδ T cell responses to respiratory BCG vaccination.
Guerra-Maupome M, McGill JL.
Sci Rep. 2019 Nov 5;9(1):15996. doi: 10.1038/s41598-019-52565-z.
Applications: Detection of bovine IL-17A and bovine IFN gamma secreting cells by ELISPOT; Measurement of bovine IL-17A and bovine IFN gamma in bronchoalveolar lavage fluid by ELISA
Abstract
The Mycobacterium bovis Bacillus Calmette-Guerin (BCG) vaccine is administered parenterally to infants and young children to prevent tuberculosis (TB) infection. However, the protection induced by BCG is highly variable and the vaccine does not prevent pulmonary TB, the most common form of the illness. Until improved TB vaccines are available, it is crucial to use BCG in a manner which ensures optimal vaccine performance. Immunization directly to the respiratory mucosa has been shown to promote greater protection from TB in animal models. γδ T cells play a major role in host defense at mucosal sites and are known to respond robustly to mycobacterial infection. Their positioning in the respiratory mucosa ensures their engagement in the response to aerosolized TB vaccination. However, our understanding of the effect of respiratory BCG vaccination on γδ T cell responses in the lung is unknown. In this study, we used a calf model to investigate the immunogenicity of aerosol BCG vaccination, and the phenotypic profile of peripheral and mucosal γδ T cells responding to vaccination. We observed robust local and systemic M. bovis-specific IFN-γ and IL-17 production by both γδ and CD4 T cells. Importantly, BCG vaccination induced effector and memory cell differentiation of γδ T cells in both the lower airways and peripheral blood, with accumulation of a large proportion of effector memory γδ T cells in both compartments. Our results demonstrate the potential of the neonatal calf model to evaluate TB vaccine candidates that are to be administered via the respiratory tract, and suggest that aerosol immunization is a promising strategy for engaging γδ T cells in vaccine-induced immunity against TB.
Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves.
McGill JL, Kelly SM, Guerra-Maupome M, Winkley E, Henningson J, Narasimhan B, Sacco RE.
Sci Rep. 2019 Oct 22;9(1):15157. doi: 10.1038/s41598-019-51684-x.
Applications: Measurement of bovine IL-17A and bovine IFN gamma in bronchoalveolar lavage fluid by ELISA
Abstract
Respiratory syncytial virus (RSV) infection is a leading cause of severe acute lower respiratory tract infection in infants and children worldwide. Vitamin A deficiency (VAD) is one of the most prevalent nutrition-related health problems in the world and is a significant risk factor in the development of severe respiratory infections in infants and young children. Bovine RSV (BRSV) is a primary cause of lower respiratory tract disease in young cattle. The calf model of BRSV infection is useful to understand the immune response to human RSV infection. We have previously developed an amphiphilic polyanhydride nanoparticle (NP)-based vaccine (i.e., nanovaccine) encapsulating the fusion and attachment proteins from BRSV (BRSV-NP). Calves receiving a single, intranasal dose of the BRSV-NP vaccine are partially protected from BRSV challenge. Here, we evaluated the impact of VAD on the immune response to the BRSV-NP vaccine and subsequent challenge with BRSV. Our results show that VAD calves are unable to respond to the mucosal BRSV-NP vaccine, are afforded no protection from BRSV challenge and have significant abnormalities in the inflammatory response in the infected lung. We further show that acute BRSV infection negatively impacts serum and liver retinol, rendering even well-nourished individuals susceptible to VAD. Our results support the use of the calf model for elucidating the impact of nutritional status on mucosal immunity and respiratory viral infection in infants and underline the importance of VA in regulating immunity in the respiratory mucosa.
Divergent Antigen-Specific Cellular Immune Responses during Asymptomatic Subclinical and Clinical States of Disease in Cows Naturally Infected with Mycobacterium avium subsp. paratuberculosis.
Stabel JR, Bannantine JP.
Infect Immun. 2019 Dec 17;88(1). pii: e00650-19. doi: 10.1128/IAI.00650-19. Print 2019 Dec 17.
Applications: Measurement of bovine IL-10, IL-12, and IL-17A in cell culture supernatants by ELISA
Abstract
Infection of the host with Mycobacterium avium subsp. paratuberculosis results in chronic and progressive enteritis that traverses both subclinical and clinical stages. The mechanism(s) for the shift from an asymptomatic subclinical disease state to advanced clinical disease is not fully understood. In the present study, naturally infected dairy cattle were divided into subclinical and clinical infection groups, along with noninfected control cows of similar parity, to study host immune responses in different stages of infection. Both infection groups had higher levels of secretion of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) than control cows, whereas only clinical cows had increased secretion of IL-10, IL-12, and IL-18 upon stimulation of peripheral blood mononuclear cells (PBMCs) with antigen. Conversely, secretion of IL-17Α was decreased for clinical cows compared to subclinical and control cows. Proinflammatory cytokine genes were upregulated only for subclinical cows, whereas increased IL-10 and IL-17 gene expression levels were observed for both infection groups. Increased CD4+, CD8+, and γδ T cell receptor-positive (TCR+) T cells were observed for subclinical cows compared to clinical cows. Although clinical cows expressed antigen-specific immune responses, the profile for subclinical cows was one of a dominant proinflammatory response to infection. We reason that a complex coordination of immune responses occurs during M. avium subsp. paratuberculosis infection, with these responses shifting as the host transitions through the different stages of infection and disease (subclinical to clinical). A further understanding of the series of events characterized by Th1/Th2/Th17 responses will provide mechanisms for disease progression and may direct insightful intervention strategies.
Interleukin 8 and Pentaxin (C-Reactive Protein) as Potential New Biomarkers of Bovine Tuberculosis.
Gao X, Guo X, Li M, Jia H, Lin W, Fang L, Jiang Y, Zhu H, Zhang Z, Ding J, Xin T.
J Clin Microbiol. 2019 Sep 24;57(10). pii: e00274-19. doi: 10.1128/JCM.00274-19. Print 2019 Oct.
Applications: Measurement of bovine IL-17A and CXCL10 (IP-10) in serum and plasma by ELISA
Prophylactic digoxin treatment reduces IL-17 production in vivo in the neonatal calf and moderates RSV-associated disease.
McGill JL, Guerra-Maupome M, Schneider S.
PLoS One. 2019 Mar 25;14(3):e0214407. doi: 10.1371/journal.pone.0214407. eCollection 2019.
Applications: Detection of bovine IL-17A and bovine IFN gamma secreting cells by ELISPOT; Measurement of bovine IL-17A and bovine IFN gamma in nasal secretions by ELISA
Abstract
Respiratory syncytial virus (RSV) is a leading cause of morbidity and mortality in human infants. Bovine RSV infection of neonatal calves is pathologically and immunologically similar to RSV infection in infants, and is therefore a useful preclinical model for testing novel therapeutics. Treatment of severe RSV bronchiolitis relies on supportive care and may include use of bronchodilators and inhaled or systemic corticosteroids. Interleukin-17A (IL-17) is an inflammatory cytokine that plays an important role in neutrophil recruitment and activation. IL-17 is increased in children and rodents with severe RSV infection; and in calves with severe BRSV infection. It is currently unclear if IL-17 and Th17 immunity is beneficial or detrimental to the host during RSV infection. Digoxin was recently identified to selectively inhibit IL-17 production by antagonizing its transcription factor, retinoid-related orphan receptor γ t (RORγt). Digoxin inhibits RORγt binding to IL-17 and Th17 associated genes, and suppresses IL-17 production in vitro in human and murine leukocytes and in vivo in rodent models of autoimmune disease. We demonstrate here that in vitro and in vivo digoxin treatment also inhibits IL-17 production by bovine leukocytes. To determine the role of IL-17 in primary RSV infection, calves were treated prophylactically with digoxin and infected with BRSV. Digoxin treated calves demonstrated reduced signs of clinical illness after BRSV infection, and reduced lung pathology compared to untreated control calves. Digoxin treatment did not adversely affect virus shedding or lung viral burden, but had a significant impact on pulmonary inflammatory cytokine expression on day 10 post infection. Together, our results suggest that exacerbated expression of IL-17 has a negative impact on RSV disease, and that development of specific therapies targeting Th17 immunity may be a promising strategy to improve disease outcome during severe RSV infection.
Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.
Benedictus L, Ravesloot L, Poppe K, Daemen I, Boerhout E, van Strijp J, Broere F, Rutten V, Koets A, Eisenberg S.
BMC Vet Res. 2019 Jan 7;15(1):15. doi: 10.1186/s12917-018-1765-9.
Applications: Measurement of bovine IL-17A in cell culture supernatants by ELISA.
Background: Staphylococcus aureus, a leading cause of mastitis in dairy cattle, causes severe mastitis and/or chronic persistent infections with detrimental effects on the cows' wellbeing, lifespan and milk production. Despite years of research there is no effective vaccine against S. aureus mastitis. Boosting of non-protective pre-existing immunity to S. aureus, induced by natural exposure to S. aureus, by vaccination may interfere with vaccine efficacy. The aim was to assess whether experimental immunization of S. aureus naïve animals results in an immune response that differs from immunity following natural exposure to S. aureus.
Results: First, to define the period during which calves are immunologically naïve for S. aureus, Efb, LukM, and whole-cell S. aureus specific serum antibodies were measured in a cohort of newborn calves by ELISA. Rising S. aureus specific antibodies indicated that from week 12 onward calves mounted an immune response to S. aureus due to natural exposure. Next, an experimental immunization trial was set up using 8-week-old heifer calves (n = 16), half of which were immunized with the immune evasion molecules Efb and LukM. Immunization was repeated after one year and before parturition and humoral and cellular immunity specific for Efb and LukM was determined throughout the study. Post-partum, antibody levels against LukM and EfB were significantly higher in serum, colostrum and milk in the experimentally immunized animals compared to animals naturally exposed to S. aureus. LukM specific IL17a responses were also significantly higher in the immunized cows post-partum.
Conclusions: Experimental immunization with staphylococcal immune evasion molecules starting before natural exposure resulted in significantly higher antibody levels against Efb and LukM around parturition in serum as well as the site of infection, i.e. in colostrum and milk, compared to natural exposure to S. aureus. This study showed that it is practically feasible to vaccinate S. aureus naïve cattle and that experimental immunization induced a humoral immune response that differed from that after natural exposure only.
Immune response after an experimental intramammary challenge with killed Staphylococcus aureus in cows and heifers vaccinated and not vaccinated with Startvac, a polyvalent mastitis vaccine.
Piepers S, Prenafeta A, Verbeke J, De Visscher A, March R, De Vliegher S.
J Dairy Sci. 2017 Jan;100(1):769-782. doi: 10.3168/jds.2016-11269. Epub 2016 Nov 3.
Applications: Measurement of Bovine IL-17A in cell culture supernatants by ELISA.
T helper 17-associated cytokines are produced during antigen-specific inflammation in the mammary gland.
Rainard P, Cunha P, Bougarn S, Fromageau A, Rossignol C, Gilbert FB, Berthon P.
PLoS One. 2013 May 16;8(5):e63471. doi: 10.1371/journal.pone.0063471. Print 2013.
Applications: Measurement by ELISA of IL-17A in bovine milk samples.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart