Swine IL-13 Polyclonal Antibody - Biotinylated
Reactivity - ELISA
Bovine IL-13 - Moderate
Canine IL-13 - Weak
Chicken IL-13 - None
Equine IL-13 - Weak
Mouse IL-13 - Weak
Swine IL-13 - Strong
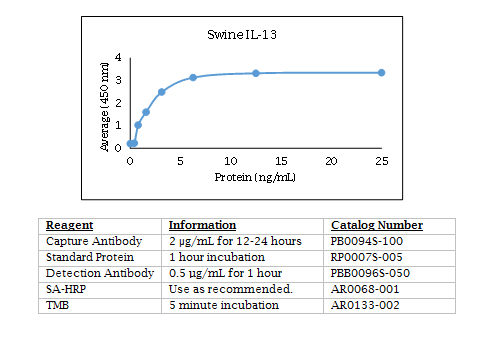
Swine IL-13 ELISA Data
Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge.
Magiri RB, Lai KJ, Mutwiri GK, Wilson HL.
Vaccines (Basel). 2020 May 18;8(2):E235. doi: 10.3390/vaccines8020235.
Applications: Enumeration of swine IL-17A and IL-13 release from lymph node cells by ELISPOT
Abstract
Vaccination is the most efficient method of protection against influenza infections. However, the rapidly mutating viruses and development of new strains make it necessary to develop new influenza vaccines annually. Hence, vaccines that stimulate cross-protection against multiple influenza subtypes are highly sought. Recent evidence suggests that adjuvants such as PCEP that promote Th1-type T cell and Th2-type T cell immune responses and broad-spectrum immune responses may confer cross-protection against heterologous influenza strains. In this study, we evaluated whether the immunogenic and protective potential of PCEP-adjuvanted inactivated swine influenza virus H1N1 vaccine can protect pigs immunized against live H3N2 virus. Piglets were vaccinated via the intradermal route with PCEP-adjuvanted inactivated swine influenza virus (SIV) H1N1 vaccine, boosted at day 21 with the same vaccines then challenged with infectious SIV H3N2 virus at day 35 via the tracheobronchial route. The pigs showed significant anti-H1N1 SIV specific antibody titres and H1N1 SIV neutralizing antibody titres, and these serum titres remained after the challenge with the H3N2 virus. In contrast, vaccination with anti-H1N1 SIV did not trigger anti-H3N2 SIV antibody titres or neutralizing antibody titres and these titres remained low until pigs were challenged with H3N2 SIV. At necropsy (six days after challenge), we collected prescapular lymph nodes and tracheobronchial draining the vaccination sites and challenge site, respectively. ELISPOTs from lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significant production of IFN-γ in the tracheobronchial cells, but not the prescapular lymph nodes. In contrast, lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significantly higher IL-13 and IL-17A in the prescapular lymph nodes draining the vaccination sites relative to unchallenged animals. Lung lesion scores show that intradermal vaccination with H1N1 SIV plus PCEP did not prevent lesions when the animals were challenged with H3N2. These results confirm previous findings that PCEP is effective as a vaccine adjuvant in that it induces strong immune responses and protects against homologous swine influenza H1N1 virus, but the experimental H1N1 vaccine failed to cross-protect against heterologous H3N2 virus.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart